Vapour Pressure and Phase Change
Vapour Pressure and Phase Change: Overview
This topic covers concepts such as Phase Diagram, Sublimation of Substances, Saturated Vapour Pressure, Boiling Point, and Triple Point.
Important Questions on Vapour Pressure and Phase Change
Superheating of water will be possible at which one of the following temperatures?
Which of the following is NOT a method to prevent superheating of a liquid?
Explain how do superheating and supercooling occur in water.
The process of boiling a liquid above its boiling point is called _____.
Boiling point of a liquid depends on its pressure.
Which one of the following relations are used to find saturated vapor pressure at room temperature?
Explain in brief why unsaturated vapor pressure decreases with temperature.
Determine the boiling point of water at vapor pressure equal to in
The normal boiling point of water at vapour pressure is , Enthalpy of vaporization for water is . Take
Which one of the following is the correct Clausius-Claperyon relation to determine the boiling point of a liquid at a given vapor pressure.
is the enthalpy of vaporization
is the universal gas constant
is the normal boiling point
is the boiling point which is to be determined
is the pressure at normal boiling point
is the pressure at which boiling point is to be determined
What is the effect of superheating of liquid on a refrigeration cycle?
Unsaturated vapour follows
Which one of the following relations are used to characterize discontinuous phase transition between two states of matter of a single constituent?
Vapour that remains in contact with its liquid surface within a closed space is called _____.
(Choose from: saturated vapour/unsaturated vapour)
What is the critical temperature?
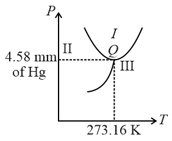
What is the triple point of water is
What is boiling point of liquid?
In the phase diagram shown, the point corresponds to the triple point of water. The regions , , and respectively corresponds to phases:
The pair of boiling point and compound are given as,
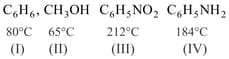
Which will show lowest vapour pressure at room temperature?
The best method for the separation of naphthalene and benzoic acid from their mixture is
Using the following information determine the boiling point of a mixture contains 1560 g benzene and 1125 g chlorobenzene, when the external pressure is 1000 torr. Assume the solution is ideal.
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
